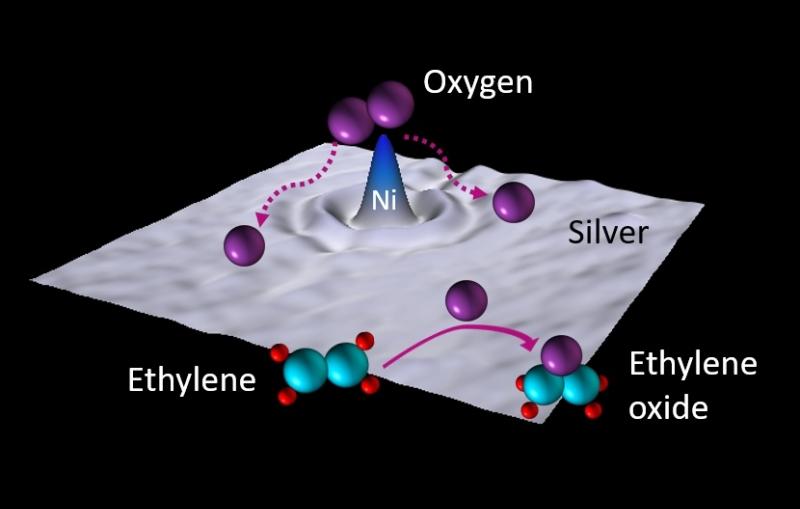
Capturing the behavior of single-atom catalysts on the move
Scientists precisely control where single-atom catalysts sit on their support structures, and show how changing their position affects their reactivity.
By Glennda Chui
Scientists are excited by the prospect of stripping catalysts down to single atoms. Attached by the millions to a supporting surface, they could offer the ultimate in speed and specificity.
Now researchers have taken an important step toward understanding single-atom catalysts by deliberately tweaking how they’re attached to the surfaces that support them – in this case the surfaces of nanoparticles. They attached one platinum atom to each nanoparticle and observed how changing the chemistry of the particle’s surface and the nature of the attachment affected how keen the atom was to catalyze reactions.
Key experiments for the study took place at the Department of Energy’s SLAC National Accelerator Laboratory, and the results were reported in Nature Materials yesterday.
“We believe this is the first time the reactivity of a metallic single-atom catalyst has been traced to a specific way of attaching it to a particular supporting structure. This study is also unique in systematically controlling that attachment,” said Simon R. Bare, a distinguished staff scientist at SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL) and a co-author of the study.
“This is an important scientific breakthrough, and understanding on a fundamental level how the structure relates to the reactivity will ultimately allow us to design catalysts to be much more efficient. There is a huge number of people working on this problem.”
Harsh treatment, good results
Bare and other SLAC scientists were part of a previous study at SSRL that found that individual iridium atoms could catalyze a particular reaction up to 25 times more efficiently than the iridium nanoparticles used today, which contain 50 to 100 atoms.
This latest study was led by Associate Professor Phillip Christopher of the University of California, Santa Barbara. It looked at individual atoms of platinum that were attached to separate nanoparticles of titanium dioxide in his lab. While this approach would probably not be practical in a chemical plant or in your car’s catalytic converter, it did give the research team exquisitely fine control of where the atoms were placed and of the environment immediately around them, Bare said.
Researchers gave the nanoparticles chemical treatments – either harsh or mild – and used SSRL’s X-rays to observe how those treatments changed where and how the platinum atoms attached to the surface.
Meanwhile, scientists at the University of California, Irvine directly observed the attachments and positions of the platinum atoms with electron microscopes, and researchers at UC-Santa Barbara measured how active the platinum atoms were in catalyzing reactions.
Breaking through the surface

A platinum atom has six binding sites where it can hook up with other atoms. In untreated nanoparticles, the atoms were buried in the surface and firmly bound to six oxygen atoms each; they had no free binding sites that could grab other atoms and start a catalytic reaction.
In mildly treated particles, the platinum atoms emerged from the surface and were bound to just four oxygen atoms apiece, leaving them two free binding sites and the potential for more catalytic activity.
And in harshly treated particles, the atoms clung to the surface by only two bonds, leaving four binding sites free. When the researchers tested the ability of the variously treated nanoparticles to catalyze a reaction where carbon monoxide combines with oxygen to form carbon dioxide – the same reaction that takes place in a car’s catalytic converter – this one came out on top, Bare said, with five times greater activity than the others.
“While this study shows the importance of understanding the dynamic nature of catalysts,” Christopher said, “the next challenge will be to translate the findings to industrially relevant systems.”
SSRL is a DOE Office of Science user facility. The changing positions of the platinum atoms on the particle surfaces were imaged and observed with transmission electron microscopy using state-of-the-art facilities recently established at the Irvine Materials Research Institute (IMRI) at UC-Irvine. Detailed experimental insights obtained in the study were correlated with predictions made by theorists at the University of Milano-Bicocca in Italy.
Citation: DeRita et al., Nature Materials, 22 April 2019 (10.1038/s41563-019-0349-9)
For questions or comments, contact the SLAC Office of Communications at communications@slac.stanford.edu.
SLAC is a multi-program laboratory exploring frontier questions in photon science, astrophysics, particle physics and accelerator research. Located in Menlo Park, Calif., SLAC is operated by Stanford University for the U.S. Department of Energy's Office of Science.
SLAC National Accelerator Laboratory is supported by the Office of Science of the U.S. Department of Energy. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time.